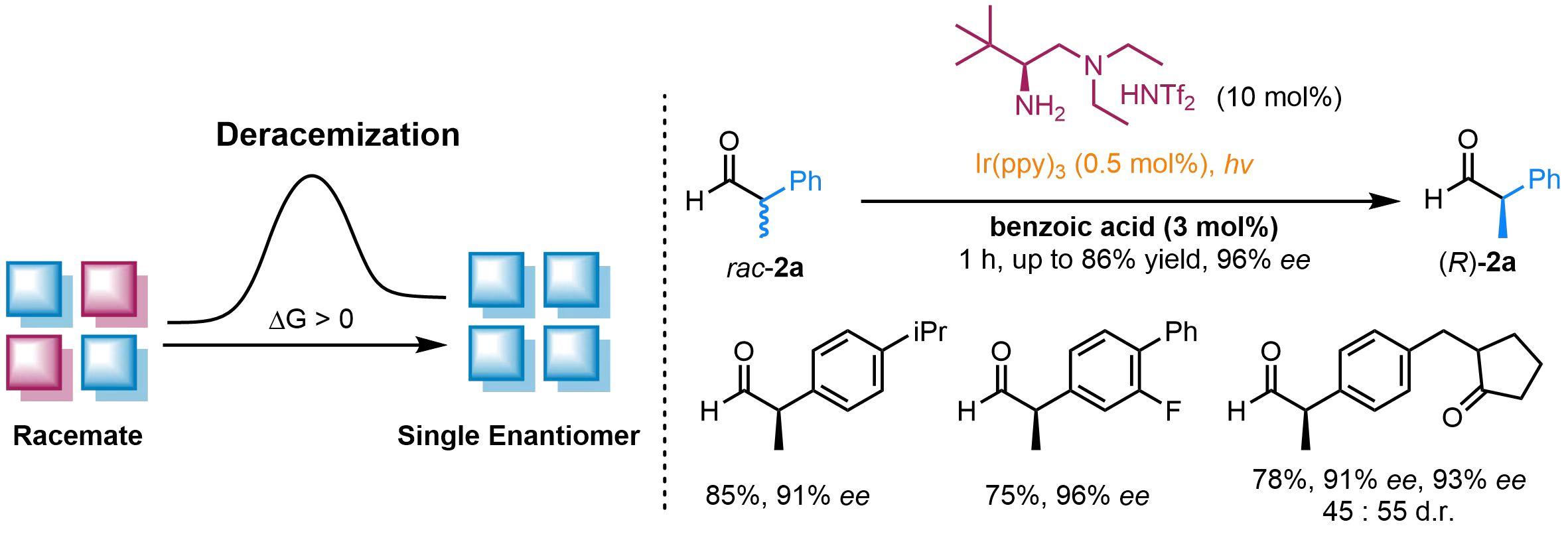
A study @ScienceMagazine by Luo Sanzhong's group from the Department of Chemistry has realized the deracemization from racemic α-branched aldehydes to their optically pure enantiomer through photochemical E/Z isomerization of enamine intermediate.

Catalytic deracemization of α-branched aldehydes is a direct strategy to construct enantiopure α-tertiary carbonyls, which are essential to pharmaceutical applications. Here, Prof. Luo Sanzhong's group report a photochemical E/Z isomerization strategy for the deracemization of α-branched aldehydes by using simple aminocatalysts and readily available photosensitizers. A variety of racemic α-branched aldehydes could be directly transformed into either enantiomer with high selectivity. Rapid photodynamic E/Z isomerization and highly stereospecific iminium/enamine tautomerization are two key factors that underlie the enantioenrichment. This study presents a distinctive photochemical E/Z isomerization strategy for externally tuning enamine catalysis.