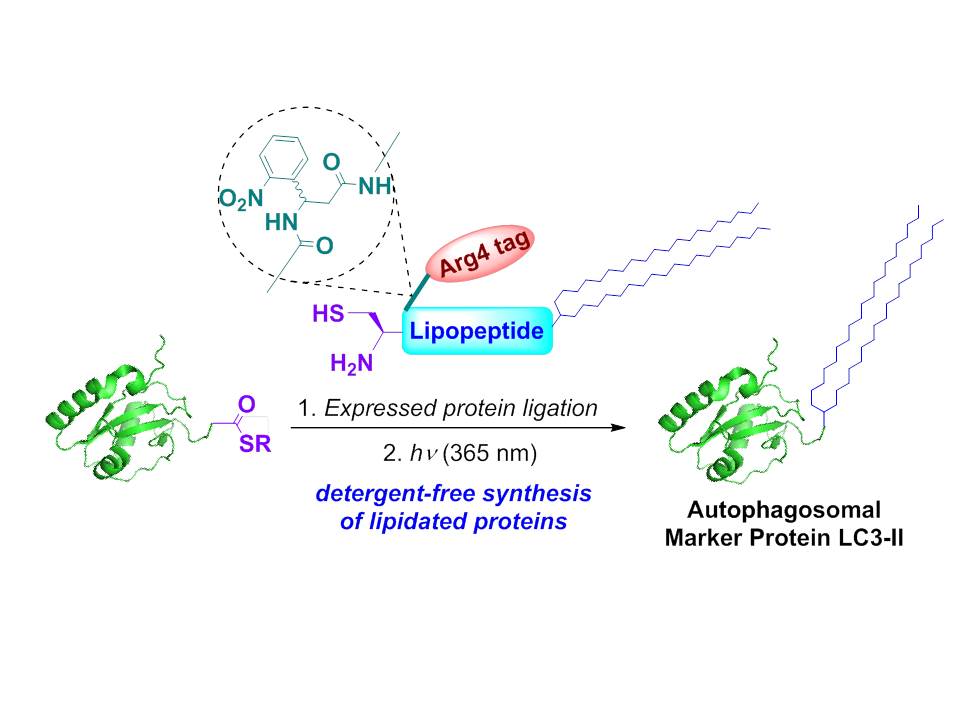
Autophagy is a catabolic process for the bulk degradation of intracellular materials. Cytoplasmic components are engulfed by the double-membrane-bound autophagosomes, and autophagosomes then fuse with lysosomes where the sequestered materials are degraded by the lysosomal hydrolases. Previous studies have shown that LC3-II is an important protein marker for the study of the machinery of autophagy. Therefore studies on autophagy require preparative amounts of LC3-II. Previous attempts to produce LC3-II using in vitro enzymatic approaches can only afford LC3-II in the low microgram scale.
Prof. Lei Liu of the Department of Chemistry, Tsinghua University recently reported the chemical synthesis of homogeneous LC3-II in practical quantities. Physicochemical and biological tests verified that the synthetic proteins had the desired structure and activity. The researchers have recognized that LC3-II, which is a lipidated protein, presents a challenging synthetic target because its lipid tail is too hydrophobic for the handlings. To solve this problem, they developed a new method using a removable solublizing side chain to assist the ligation of the lipopeptides. This strategy may provide a general approach for the synthesis of lipidated proteins. Please refer Angew. Chem. Int. Ed. 2013, 52, 4858 to see the details.