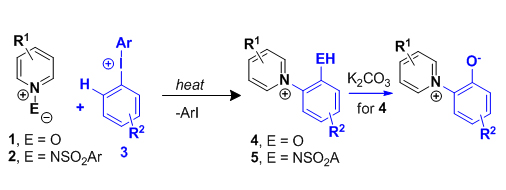
Orth-disubstituted benzene derivatives are key scaffolds in a number of optical materials and natural products as well as the important intermediates in organic sythesis. Owing to the directing and steric effects, orth-disubstituted benzene and their derivatives are generally tough to obtain and tradionally prepared by multi-step procedures. By utilizing easily obtainable and environmentally friendly hypervalent iodine reagents as starting material, Chao Chen's group from the department of chemistry have recently established a new way of selectivly sythesizing orth- disubstuted benzene derivatives. Via a 1,3-radical rearrangement, reaction of diaryliodonium salts with pyridine N-oxides took place and thus generated o-pyridinium phenolates with high-selectivity. Futher investigation has shown that diaryliodonium salts reacted with N-amidates simlarly via a 1,3-radical rearrangement and o-pyridinium anilinates were isolated with high-selectivity. Such sythetic route is easily operated without any metal catalysts while the starting materials are conveniently accessble and the desired products are isolated in high yield. It proved to be a quite efficient approach to multi-substituted benzene derivatives for its tolerance with various functional groups.
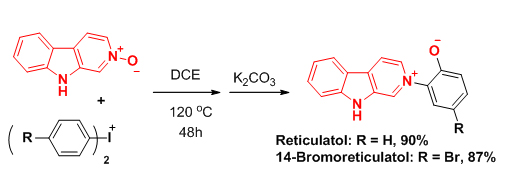
Under the optimized condition, natural product Reticulatol with anti-leukemia activity for the first time has been successfully synthesized. This research work has been published on Angew. Chem. Int. Ed., 2013, 52, 7574.