Bolaamphiphile, possessing a specific bipolar structure, is a useful building blocks for fabricating assemblies with high thermal stability and various self-assembled structures. Although diversified natural or artificial bolaamphiphiles with tailor-made architectures and features have been documented, controlling the self-assembly of bolaamphiphiles in a facile manner still remains a big challenge.
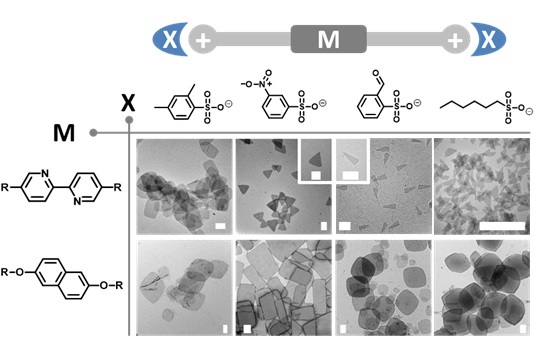
Recently, Prof. Xi Zhang’s group has demonstrated that tosylate ion as counteranion for cationic bolaamphiphiles could generally lead to the formation of two-dimensional (2D) planar aggregates, while common counteranions like halide ions could only lead to one-dimensional or zero-dimensional aggregates. As tosylate ion belongs to a class of ions denoted as hydrotropic anions, possessing both a hydrophobic part and a hydrophilic-polar-head, Zhang and his coworkers subsequently test around 60 various hydrotropic counteranions, and reveal that the ability of a hydrotropic counteranion to induce the formation of 2D planar aggregates depends weakly on its polar head, but is strongly correlated to the size and the substitution pattern of its organic portion.
Additionally, 2D planar aggregates with various shapes such as triangle, quadrangle, and hexagon can be modulated both by counteranions and the embedded conjugated moieties, which may provide a simple and feasible methodology for fabricating 2D organic self-assembled structures with controlled features.
These integrated works were accomplished by the cooperation with Prof. Mario Smet in Katholieke Universiteit Leuven, Belgium and Dr. Charl F. J. Faul in University of Bristol, UK, and have led to two edge articles in Chemical Science. (Chem. Sci., 2013, 4, 4486-4493, Chem. Sci., 2014, DOI: 10.1039/C4SC00860J).