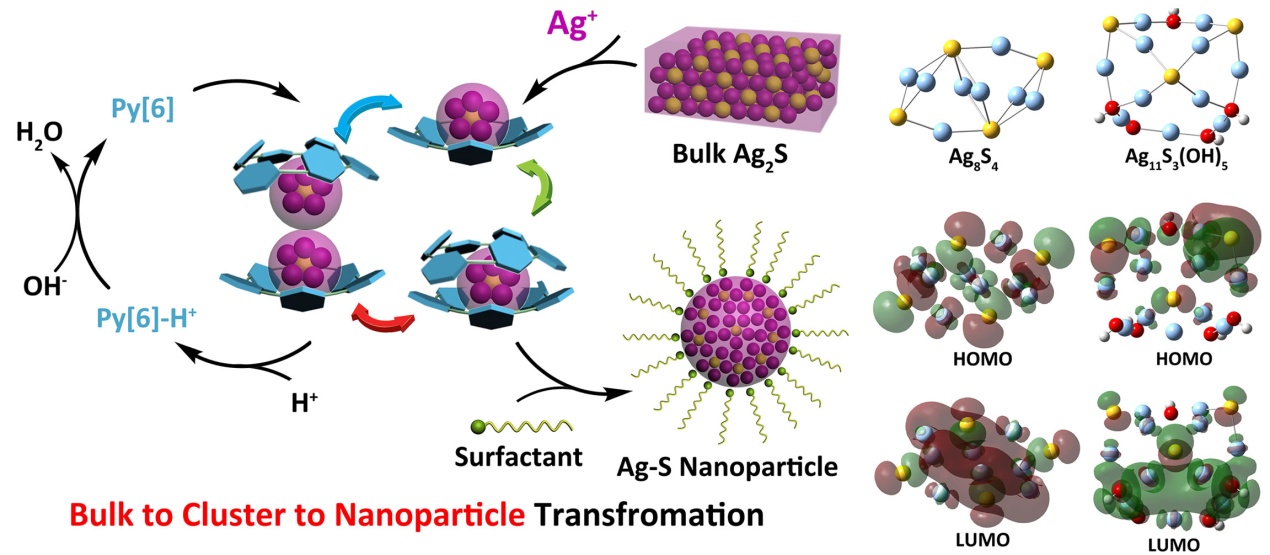
Multi-nuclear metal clusters, which have long been regarded as important intermediates of synthesizing nano-compounds, have significant impact on the properties of resulting nanomaterials by varying their compositions and structures. With the financial support from National Natural Science Foundation of China and Ministry of Science and Technology, Liang Zhao and co-workers from Tsinghua University took advantage of strong coordination ability of multi-dentate azacalixpyridine macrocycles to undertake a “top-down” mild synthesis of silver-sulfide clusters by using indissolvable bulk Ag2S (Ksp (Ag2S, 25 ºC) = 8*10-51) as starting material. After deprotection of the cluster by adding acid to remove macrocycles, the released Ag-S clusters mutually coalesced to produce silver sulfide nanoparticles through a “bottom-up” route. The acidified macrocycles could be recycled by subsequent separation, neutralization and extraction. Such macrocycle-assisted nanoparticlization method yielded a new kind of Ag-S nanoparticles inherent with high silver to sulphide ratio (3.5:1) which was difficult to access based on traditional synthetic approaches. In addition, the as-prepared Ag-S nanoparticles possess a larger band gap energy than those made by conventional ways. In addition, they cooperated with Hong Jiang from Peking University to reveal the influence of Ag/S ratio on HOMO-LUMO molecular orbitals by using Hybrid-DFT calculations. This work offers an alternative way to prepare functional materials through supramolecular self-assembly methods. This work was published as an Edge Article on Chemical Science (X. He, Y. Wang, C.-Y. Gao, H. Jiang and L. Zhao*, Chem. Sci. 2014, DOI: 10.1039/C4SC01884B).