Supported by the National Natural Science Foundation of China and Ministry of Science and Technology of the People's Republic of China, Dr. Huaping Xu and Prof. Xi Zhang from Tsinghua University have done systematic researches on the selenium-containing functional materials (Figure 1). They firstly reported the dual redox and gamma-irradiation behaviors of diselenide-containing block copolymers assemblies (J. Am. Chem. Soc., 2010, 132, 442-443), revealed the possibility of their use as vehicles in a combination of chemotherapy and actinotherapy (Langmuir, 2011, 27, 5874–5878). They also describe the amphiphilic monoselenide containing block copolymers which possesses the reversible oxidation and reduction properties (Soft Matter, 2012, 8, 1460-1466). In addition, based on the non-covalent interactions such as electrostatic interaction, controlled self-assembly and disassembly of selenium-containing polymeric supra-amphiphile can be achieved by oxidation and reduction (Langmuir, 2010, 26, 14414-14418). They were invited to contribute a review article in Accounts of Chemical Research (2013, ASAP, DOI: 10.1021/ar4000339).
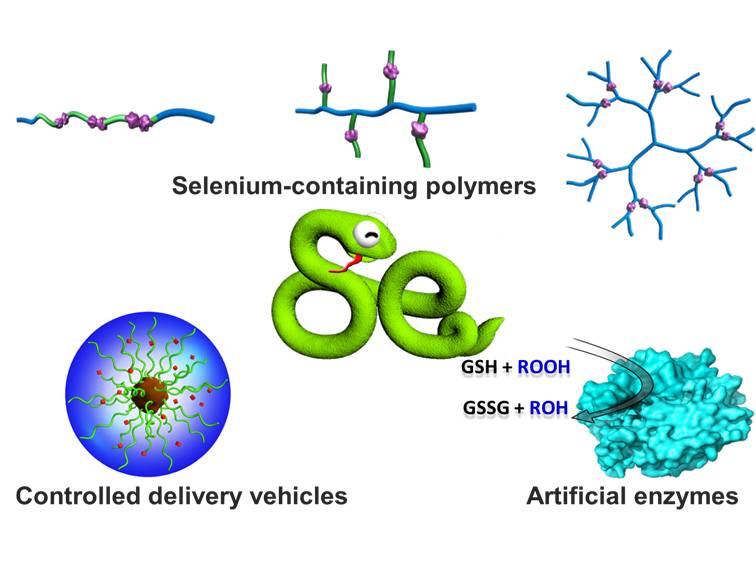
Figure 1. Selenium-containing polymers: promising biomaterials for controlled release and enzyme mimics.
Recently, they serendipitously found a kind of Se-N covalent bond with dynamic property when they were studying the controlled self-assembly and disassembly of the selenium-containing block copolymers (Figure 2). This kind of Se-N dynamic covalent bond is formed between the Se atom of a phenylselenyl halogen species and the N atom of a pyridine derivative with sonication, which can be reversibly and rapidly formed or cleaved under acidic or basic conditions, respectively. Furthermore, the Se-N bond can be dynamically cleaved by heating or treatment with stronger electron-donating pyridine derivatives. This kind of Se-N covalent bond is a new kind of dynamic covalent bond which can provide new driving forces for supramolecular self-assembly, and it may find applications in constructing reversible and recycle functional supramolecular materials and polymers (Chem. Eur. J 2013, DOI: 10.1002/chem.201301446).
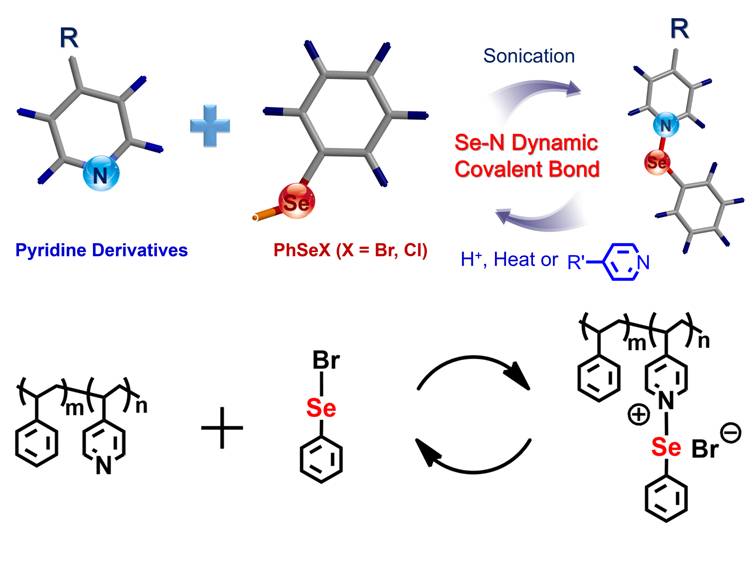
Figure 2. Se-N: a new dynamic covalent bond.