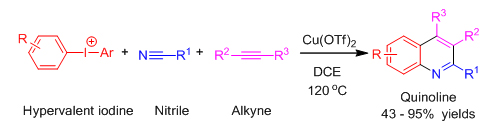
Nitrogenous heterocycles such as quinolines and quinazolines are privileged scaffolds in many organic optoelectronic materials,medical agents and natural products. Traditionally,nitrogenous heterocycles compounds are prepared with toxic anilines via condensation reactions, substitution reactions with carbonyl compounds, where a large amout of greenhouse gases and waste would be generated. Consequently, it is crucial to develop new approaches towards nitrogenous heterocycles with green and ecological starting materials instead of anilines. Chao Chen's reasearch group has reported a brand new method of sythesizing quinoline ring scaffolds by using easily obtainable hypervalent iodine compounds, nitriles and alkynes as starting material. While catalyzing by copper, a series of quinolines and their derivatives were prepared. Such three-component and one-pot reactions are conveniently conducted and suitable for sythesizing quinolines and their derivatives with muti-functional groups, and thus would hopefully replace the traditional synthesis methods with anilines. This research work has been published on Angew. Chem. Int. Ed., 2013, 52, 5323.
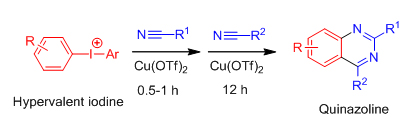
Inspired by the same strategy, Chao Chen's reasearch group has also successfully prepared a series of quinazolines and their derivatives via hypervalent iodine compouds with two equivalent nitriles. This research work has been published on Chem. Commun., 2013, 49, 6752.